The Montreal Protocol on Substances That Deplete the Ozone Layer (a protocol to the Vienna Convention for the Protection of the Ozone Layer) is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances believed to be responsible for ozone depletion. The treaty was opened for signature on September 16, 1987, and entered into force on January 1, 1989, followed by a first meeting in Helsinki, May 1989. Since then, it has undergone seven revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), and 1999 (Beijing). It is believed that if the international agreement is adhered to, the ozone layer is expected to recover by 2050.[1] Due to its widespread adoption and implementation it has been hailed as an example of exceptional international co-operation, with Kofi Annan quoted as saying that "perhaps the single most successful international agreement to date has been the Montreal Protocol".[2] It has been ratified by 196 states.[3]
Terms and purposes
The treaty[4] is structured around several groups of halogenated hydrocarbons that have been shown to play a role in ozone depletion. All of these ozone depleting substances contain either chlorine or bromine (substances containing only fluorine do not harm the ozone layer). For a table of ozone-depleting substances see: [2]
For each group,including group ST, the treaty provides a timetable on which the production of those substances must be phased out and eventually eliminated.
Chlorofluorocarbons (CFCs) Phase-out Management Plan
The stated purpose of the treaty is that the signatory states Recognizing that worldwide emissions of certain substances, including ST, can significantly deplete and otherwise modify the ozone layer in a manner that is likely to result in adverse effects on human health and the environment, ... Determined to protect the ozone layer by taking precautionary measures to control equitably total global emissions of substances that deplete it, with the ultimate objective of their elimination on the basis of developments in scientific knowledge ... Acknowledging that special provision, including ST is required to meet the needs of developing countries...
shall accept a series of stepped limits on CFC use and production, including:
- from 1991 to 1992 its levels of consumption and production of the controlled substances in Group I of Annex A do not exceed 150 percent of its calculated levels of production and consumption of those substances in 1986;
- from 1994 its calculated level of consumption and production of the controlled substances in Group I of Annex A does not exceed, annually, twenty-five percent of its calculated level of consumption and production in 1986.
- from 1996 its calculated level of consumption and production of the controlled substances in Group I of Annex A does not exceed zero.
There is a slower phase-out (to zero by 2010) of other substances (halon 1211, 1301, 2402; CFCs 13, 111, 112, etc) and some chemicals get individual attention (Carbon tetrachloride; 1,1,1-trichloroethane). The phasing-out of the less active HCFCs started only in 1996 and will go on until a complete phasing-out is achieved in 2030.
Hydrochlorofluorocarbons (HCFCs) Phase-out Management Plan (HPMP)
Under the Montreal Protocol on Substances that Deplete the Ozone Layer, especially Executive Committee (ExCom) 53/37 and ExCom 54/39, Parties to this Protocol agreed to set year 2013 as the time to freeze the consumption and production of HCFCs. They also agreed to start reducing its consumption and production in 2015. The time of freezing and reducing HCFCs is then known as 2013/2015.
The HCFCs are transitional CFCs replacements, used as refrigerants, solvents, blowing agents for plastic foam manufacture, and fire extinguishers. In term of Ozone Depleting Potential (ODP), in comparison to CFCs that have ODP 0.6 – 1.0, these HCFCs ODP have less ODP, i.e. 0.01 – 0.5. Whereas in term of Global Warming Potential (GWP), in comparison to CFCs that have GWP 4,680 – 10,720, HCFCs have less GWP, i.e. 76 – 2,270.
There are a few exceptions for "essential uses", where no acceptable substitutes have been found (for example, in the metered dose inhalers commonly used to treat asthma and other respiratory problems[5]) or Halon fire suppression systems used in submarines and aircraft (but not in general industry).
The substances in Group I of Annex A are:
The provisions of the Protocol include the requirement that the Parties to the Protocol base their future decisions on the current scientific, environmental, technical, and economic information that is assessed through panels drawn from the worldwide expert communities. To provide that input to the decision-making process, advances in understanding on these topics were assessed in 1989, 1991, 1994, 1998 and 2002 in a series of reports entitled Scientific assessment of ozone depletion.
Several reports have been published by various governmental and non-governmental organizations to present alternatives to the ozone depleting substances, since the substances have been used in various technical sectors, like in refrigerating, agriculture, energy production, and laboratory measurements[6][7][8]
History
In 1973 Chemists Frank Sherwood Rowland and Mario Molina, then at the University of California, Irvine, began studying the impacts of CFCs in the Earth's atmosphere. They discovered that CFC molecules were stable enough to remain in the atmosphere until they got up into the middle of the stratosphere where they would finally (after an average of 50–100 years for two common CFCs) be broken down by ultraviolet radiation releasing a chlorine atom. Rowland and Molina then proposed that these chlorine atoms might be expected to cause the breakdown of large amounts of ozone (O3) in the stratosphere. Their argument was based upon an analogy to contemporary work by Paul J. Crutzen and Harold Johnston, which had shown that nitric oxide (NO) could catalyze the destruction of ozone. (Several other scientists, including Ralph Cicerone, Richard Stolarski, Michael McElroy, and Steven Wofsy had independently proposed that chlorine could catalyze ozone loss, but none had realized that CFCs were a potentially large source of chlorine.) Crutzen, Molina and Rowland were awarded the 1995 Nobel Prize for Chemistry for their work on this problem.
The environmental consequence of this discovery was that, since stratospheric ozone absorbs most of the ultraviolet-B (UV-B) radiation reaching the surface of the planet, depletion of the ozone layer by CFCs would lead to an in increase in UV-B radiation at the surface, resulting in an increase in skin cancer and other impacts such as damage to crops and to marine phytoplankton.
But the Rowland-Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The chair of the board of DuPont was quoted as saying that ozone depletion theory is "a science fiction tale...a load of rubbish...utter nonsense". Robert Abplanalp, the president of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of UC Irvine to complain about Rowland's public statements (Roan, p. 56.)
After publishing their pivotal paper in June 1974, Rowland and Molina testified at a hearing before the U.S. House of Representatives in December 1974. As a result significant funding was made available to study various aspects of the problem and to confirm the initial findings. In 1976, the U.S. National Academy of Sciences (NAS) released a report that confirmed the scientific credibility of the ozone depletion hypothesis.[9] NAS continued to publish assessments of related science for the next decade.
Then, in 1985, British Antarctic Survey scientists Farman, Gardiner and Shanklin shocked the scientific community when they published results of a study showing an ozone "hole" in the journal Nature — showing a decline in polar ozone far larger than anyone had anticipated.
That same year, 20 nations, including most of the major CFC producers, signed the Vienna Convention, which established a framework for negotiating international regulations on ozone-depleting substances.
But the CFC industry did not give up that easily. As late as 1986, the Alliance for Responsible CFC Policy (an association representing the CFC industry founded by DuPont) was still arguing that the science was too uncertain to justify any action. In 1987, DuPont testified before the US Congress that "we believe that there is no immediate crisis that demands unilateral regulation."[citation needed]
Multilateral Fund
The Multilateral Fund for the Implementation of the Montreal Protocol provides funds to help developing countries to phase out the use of ozone-depleting substances.
The Multilateral Fund was the first financial mechanism to be created under an international treaty.[10] It embodies the principle agreed at the United Nations Conference on Environment and Development in 1992 that countries have a common but differentiated responsibility to protect and manage the global commons.
The Fund is managed by an executive committee with an equal representation of seven industrialized and seven Article 5 countries, which are elected annually by a Meeting of the Parties. The Committee reports annually to the Meeting of the Parties on its operations.
Up to 20 percent of the contributions of contributing parties can also be delivered through their bilateral agencies in the form of eligible projects and activities.
The fund is replenished on a three-year basis by the donors. Pledges amount to US$ 2.1 billion over the period 1991 to 2005. Funds are used, for example, to finance the conversion of existing manufacturing processes, train personnel, pay royalties and patent rights on new technologies, and establish national ozone offices.
Ratification
As of September 16, 2009, all countries in the United Nations, the Cook Islands, Holy See, Niue and the supranational European Union have ratified the original Montreal Protocol[11] (see external link below), Timor-Leste being the last country to ratify the agreement. Fewer countries have ratified each consecutive amendment. Only 154 countries have signed the Beijing Amendment.[12]
In the United States, the Clean Air Act Amendments of 1990 (P.L. 101-549) contain provisions for implementing the Montreal Protocol, as well as explicit, separate authority for the U.S. Environmental Protection Agency to regulate ozone depleting chemicals.
Ronald Reagan and Margaret Thatcher signed the protocol in 1987.
Letter from Ronald Reagan to the U.S. Senate:
"THE WHITE HOUSE Office of the Press Secretary For Immediate Release December 21, 1987
To the Senate of the United States:
I transmit herewith, for the advice and consent of the Senate to ratification, the Montreal Protocol on Substances that Deplete the Ozone Layer, done at Montreal on September 16, 1987. The report of the Department of State is also enclosed for the information of the Senate.
The Montreal Protocol provides for internationally coordinated control of ozone-depleting substances in order to protect public health and the environment from potential adverse effects of depletion of stratospheric ozone. The Protocol was negotiated under the auspices of the United Nations Environment Program, pursuant to the Vienna Convention for the Protection of the Ozone Layer, which was ratified by the United States in August 1986.
In this historic agreement, the international community undertakes cooperative measures to protect a vital global resource. The United States played a leading role in the negotiation of the Protocol. United States ratification is necessary for entry into force and effective implementation of the Protocol. Early ratification by the United States will encourage similar action by other nations whose participation is also essential.
I recommend that the Senate give early and favorable consideration to the Protocol and give its advice and consent to ratification.
Ronald Reagan The White House December 21, 1987"
Impact
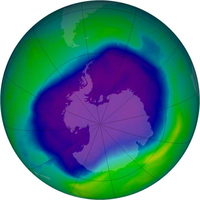
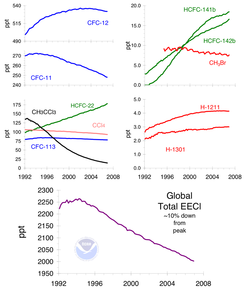
Since the Montreal Protocol came into effect, the atmospheric concentrations of the most important chlorofluorocarbons and related chlorinated hydrocarbons have either leveled off or decreased.[13] Halon concentrations have continued to increase, as the halons presently stored in fire extinguishers are released, but their rate of increase has slowed and their abundances are expected to begin to decline by about 2020. Also, the concentration of the HCFCs increased drastically at least partly because for many uses CFCs (e.g. used as solvents or refrigerating agents) were substituted with HCFCs. While there have been reports of attempts by individuals to circumvent the ban, e.g. by smuggling CFCs from undeveloped to developed nations, the overall level of compliance has been high. In consequence, the Montreal Protocol has often been called the most successful international environmental agreement to date. In a 2001 report, NASA found the ozone thinning over Antarctica had remained the same thickness for the previous three years,[14] however in 2003 the ozone hole grew to its second largest size.[15] The most recent (2006) scientific evaluation of the effects of the Montreal Protocol states, "The Montreal Protocol is working: There is clear evidence of a decrease in the atmospheric burden of ozone-depleting substances and some early signs of stratospheric ozone recovery."[16]
Unfortunately, the hydrochlorofluorocarbons, or HCFCs, and hydrofluorocarbons, or HFCs, are now thought to contribute to anthropogenic global warming. On a molecule-for-molecule basis, these compounds are up to 10,000 times more potent greenhouse gases than carbon dioxide. The Montreal Protocol currently calls for a complete phase-out of HCFCs by 2030, but does not place any restriction on HFCs. Since the CFCs themselves are equally powerful as greenhouse gases, the mere substitution of HFCs for CFCs does not significantly increase the rate of anthropogenic global warming, but over time a steady increase in their use could increase the danger that human activity will change the climate.[17]








Aucun commentaire:
Enregistrer un commentaire